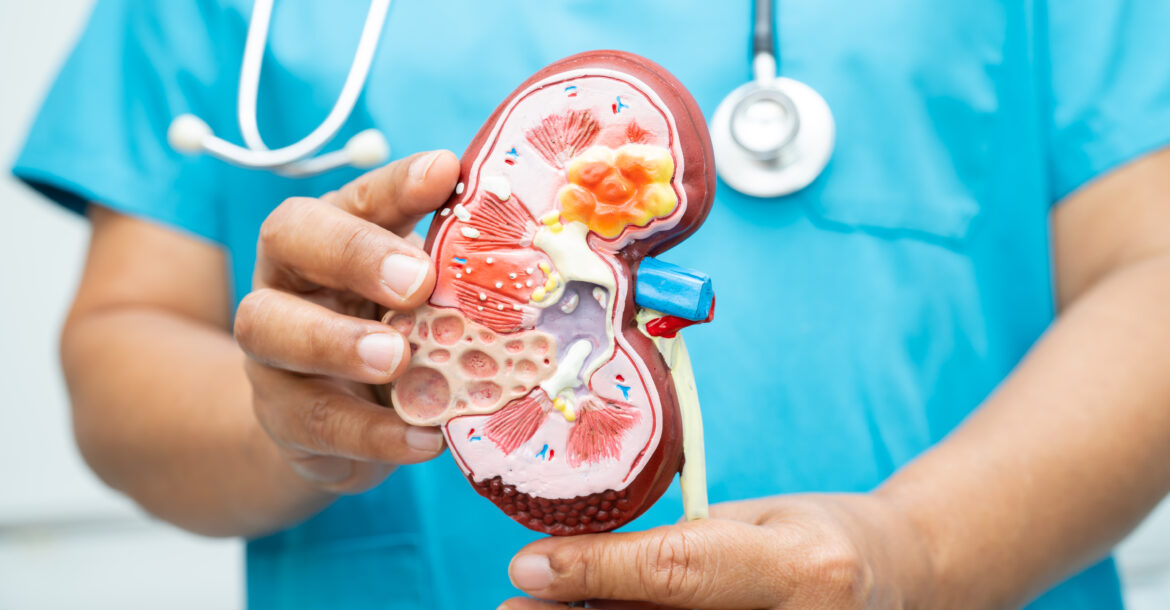
Washington: The approval is based on a late-stage trial showing that Ozempic slashed the risk of severe kidney outcomes—including kidney failure, declining kidney function, or death from kidney or heart complications—by 24% compared with a placebo.
With 40% of Type 2 diabetes patients affected by CKD, the FDA’s decision provides a new lifeline for those at risk of kidney failure and cardiovascular disease, two of the leading causes of death in the U.S.
Patients who took Ozempic in the trial also saw:
Read More
- Childhood high blood pressure doubles in 20 years as obesity crisis deepens, 114 million children affected
- Medical team performs Oman’s first artificial heart transplant at Royal Hospital
- 38-year-old Nigerian patient with rare skull base tumour successfully treated at Aster Royal Al Raffah Hospital in complex 11-hour surgery
- Royal Hospital marks milestone with Oman’s first robotic surgery
- Walk your way to better health and happiness
18% reduction in major cardiovascular events like heart attacks.
20% lower risk of death from any cause.
29% drop in cardiovascular-related deaths.
The decision is expected to reshape how doctors manage CKD in diabetic patients, offering a proven therapy to slow disease progression and improve survival rates.